Abstract
Background: Extranodal natural killer (NK)/T-cell lymphoma (ENKTL) is a rare aggressive type of non-Hodgkin's lymphoma associated with Epstein-Barr virus infection. Relapse or refractory (RR)-ENKTL cases are characterized by a high rate of relapse and dismal prognosis with limited therapeutic options. Immune check point inhibitors (ICIs) represent the cornerstone of treatment for RR-ENKTL; however, limited information is available of a rechallenge with ICIs after ICIs are discontinued due to disease progression. Epigenetic aberrations are critical as genetic abnormalities is the hallmarks of ENKTL. One of current promising strategies is that combining epigenetic and ICIs can overcome tumor resistance and enhance immunotherapy responses. Here, we assess the efficacy and safety of DNA methyltransferases inhibitors (DNMTi) plus anti-PD-1 therapy in RR-ENKTL.
Methods: This trial enrolled eligible patients with histologically confirmed RR-ENKTL failing from asparaginase-based regimen or ICIs therapy; adequate organ function and bone marrow function; and at least one measurable or evaluable lesion or symptoms. Patients received standard dosage of anti-PD-1 antibodies plus decitabine (10mg/d, d1-5) or azacytidine (100mg/d, d1-5) every 21 days until disease progression, death, intolerable toxicity. Patients accept ASCT as consolidation after they obtained CR. The primary endpoints were objective response rate (ORR) based on Lugano 2016 criteria and clinical benefit rate. Secondary endpoints were progression-free survival (PFS), overall survival (OS) and safety. Adverse events (AEs) were defined according to CTCAE 5.0.
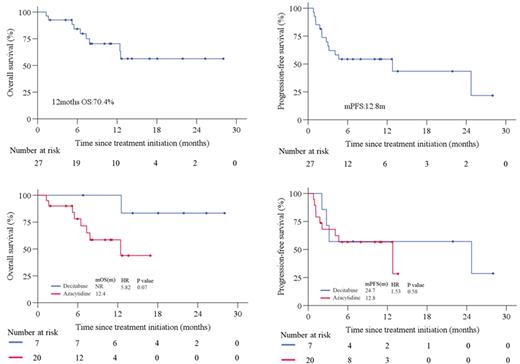
Results: From March 2020 to May 2022, 27 eligible patients were enrolled. Median age 40 years (range, 12-77), 15 (55.6%) male, 13 (48.1%) patients with stage IV of disease at screening, 11 (40.7%) patients with PINK-E score ≥ 3 points. Among them, 5(18.5%) patients had no imaging lesions, but all peripheral blood ctDNA were positive, they had high plasma EBV-DNA level(n=3), persistent fever(n=2) associated with ENKTL. The median number of prior systemic therapies was 2 (1-5), 21(77.8%)patients received ≥ 2 lines of prior systemic therapy. Twenty-two (81.5%) patients experienced prior ICIs therapy, 23(85.2%) previously received HDACi. The median time between the last dose of prior ICIs and initiation of this trial was 0.8 months (0.8 -23). Seven (25.9%) patients received decitabine, 20 (74.1%) received azacytidine. Of 22 imaging objective response evaluable patients, 13 (59.1%) achieved response (iORR) including 10 (45.5%) patients with CR (iCR). Clinical benefit analysis of 27 patients showed that clinical response(cORR) was 63.0% (17/27), 14 (59.1%) got CR (cCR). Among 5 patients with positive ctDNA alone, 4 patients achieve clinical improvement ,ctDNA negativity(n=4), EBV clearance(n=2) or fever disappearance (n=2). Among 22 patients previously received ICIs (20 patients previously received anti-PD-1 antibody plus HDACi), of these, 11 (50%) had response. After anti-PD-1 antibodies rechallenge plus DNMTi, iORR and cORR were 58.8% (10/17), 63.6% (14/22), iCR and cCR were 41.2% (7/17), 50.0% (11/22), respectively. Anti-PD-1 antibodies rechallenge plus DNMTi yielded iORR were 72.7% (8/11) and 55.6% (5/9) in patients responded to prior ICIs therapy. The difference of efficacy between decitabine and azacytidine was no significant, iORR and cORR were 60% (3/5), 58.8% (10/17) and 71.4% (5/7), 60.0% (12/20), respectively in this study. The median follow-up time was 12.1 (1.9-28.0) months, 1-year OS rate was 70.4%, median PFS rate was 12.8months. Survival results were similar with decitabine and azacytidine cohort. Sixteen (59.3%) patients reported treatment-related AEs (TRAEs). The most frequently observed (≥10%) TRAEs were lymphopenia (44.4%), neutropenia (37.0%), hypothyroidism (25.9%), nausea (18.5%), hyperglycemia (11.1%).
Conclusion: DNMTi combined with anti-PD-1 immunotherapy showed promising efficacy and manageable safety profile in RR-ENKTL for the first time. ICIs rechallenge is still an active and feasible strategy, further prospective studies are needed to clarify the role of rechallenge ICIs in this population.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.